On 9th February 2019 new regulations came into force covering the distribution of all prescription only medicines.
Manufacturers and parallel distributers are required to change their packaging to include an anti-tamper proof device, additional human readable information and include a 2-D barcode. In addition, they are required to upload the data into a Europe-wide database for verification and de-commissioning of medicine packs by end users.
Access to the European FMD database is controlled via the National Medicine Verification System (NMVS). Mysoft is a registered software supplier and has created an interface to allow verification and de-commissioning of medicine packs. The interface has successfully been deployed at a major pharmaceutical business in the UK.
The API’s (Application Programming Interface) included in the pack are as follows;
- Verify Single Pack
- Dispense Single Pack
- Undo Dispense Single Pack
- Bulk Verify Packs
- Bulk Dispense Packs
- NMVS Connection Test
As with all Mysoft interfaces our Log Viewer is included to assist with auditing of transactions.
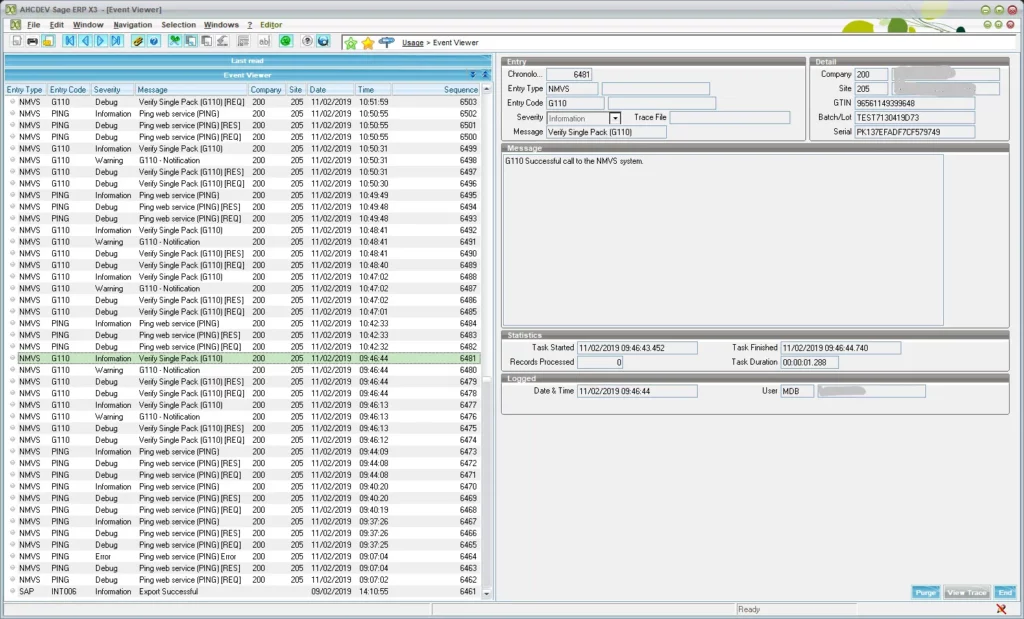
Mysoft will also provide additional services to help integrate the FMD pack into your business processes. We can also provide expertise to help set-up scanners to read 2-D barcodes.
Find out more about our expertise in the Pharmaceutical Industry, click here.
What do we do?
-
Sage X3 Advanced Telesales
Sales -
Sage X3 Carrier Interface
Distribution -
Sage X3 Diary Notes
Management -
Sage X3 E-commerce
E-commerce -
Sage X3 EDI
Finance -
Sage X3 Exchange Rate
Finance -
Sage X3 Postcode Address Look-up
Distribution -
Sage X3 Purchase Invoice Approval
Finance -
Sage X3 Reforestation As A Service (RAAS)
CSR -
Sage X3 Sales History
Sales -
Sage X3 Stock Situation
Distribution
-
Sage X3 Advanced Telesales
Sales -
Sage X3 Carrier Interface
Distribution -
Sage X3 Diary Notes
Management -
Sage X3 E-commerce
E-commerce -
Sage X3 EDI
Finance -
Sage X3 Exchange Rate
Finance -
Sage X3 Postcode Address Look-up
Distribution -
Sage X3 Purchase Invoice Approval
Finance -
Sage X3 Reforestation As A Service (RAAS)
CSR -
Sage X3 Sales History
Sales -
Sage X3 Stock Situation
Distribution
